How do the dipoles cancel despite the fact there’s an odd number of F atoms around the AsF5? What’s special in the geometry of As that makes its properties different from other molecules? This is what made me dig into the topic deeply and finally, I came up with answers to all my questions.
If you are also one who found yourself asking these questions then you are at the right place. In this article, we will get into the structure of AsF5 to find its polarity and to answer if AsF5 is polar and nonpolar. Let’s get started.
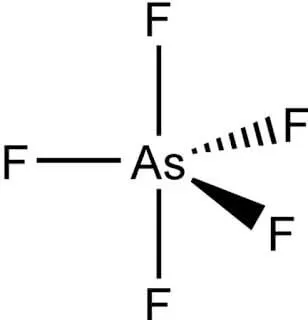
What is the Structure of AsF5
To understand the dipole nature of the molecule first we need to understand its structure. Arsenic Pentafluoride (AsF5) is a molecule that consists of one arsenic atom surrounded by five fluorine atoms. In AsF5, the molecules are shaped like a two-sided pyramid or trigonal bipyramidal. The middle is an arsenic atom surrounded by three fluorine atoms in a triangle, and two more fluorine atoms sticking out above and below.
Each fluorine atom in AsF5 is connected to the arsenic atom by one bond. There are a total of 40 valence electrons in AsF5: 5 from arsenic and 7 from each fluorine. When AsF5 is solid, the distance between the arsenic and fluorine atoms above and below is 171.9 pm, and the distance between the arsenic and the three fluorine atoms in a triangle is 166.8 pm.
Is AsF5 Polar or Nonpolar?
AsF5 has five bonds between arsenic and fluorine that seem like they should make it polar. But surprisingly, it’s actually nonpolar because of its symmetrical shape. This might raise a question: how can something with polar parts be overall nonpolar? We’ll dig into the structure and bonds of AsF5 to find out in the next section.
How is AsF5 a Nonpolar Molecule?
To see why AsF5 isn’t polar, we look at VSEPR theory and electronegativity.
AsF5 has a shape like two pyramids stuck together. It has five spots where electrons hang out (the bonds between arsenic and fluorine) and no lone pairs. There are two atoms above and below and three in the middle, arranged in a triangle.
Fluorine pulls harder on electrons than arsenic does. This makes little dipoles, or uneven pulls, toward each fluorine atom. But because of how the atoms are arranged, these dipoles balance out. The ones pointing up cancel with the ones pointing down, and the ones in the middle cancel each other too. So, the molecule as a whole doesn’t have a dipole which makes it nonpolar.
Check out the lewis structure of AsF5.
What are the Uses of AsF5?
Arsenic Pentafluoride (AsF5) has many uses in different areas:
Making Ionic Complexes
AsF5 is good at grabbing fluoride ions and forming special complexes.
Creating Superacids
When mixed with certain acids, AsF5 forms super-strong acids, which are handy in various chemical reactions.
Producing Conductive Polymers
AsF5 helps make polymers that conduct electricity. These are used in batteries, capacitors, and coatings that prevent static.
Following Regulations
AsF5 is mentioned in rules from several safety organizations, showing its importance in industry standards.
Medical Significance
Though not directly about AsF5, arsenic compounds like arsenic trioxide have been used for medicine and poison for thousands of years, since ancient times.
Don’t miss reading on Industrial Flash Module.
FAQs
How do the dipoles cancel if there is an odd number of F atoms around the AsF5?
In AsF5, the dipoles cancel out due to its symmetrical trigonal bipyramidal shape. The dipole moments of the As-F bonds cancel each other out, resulting in a net dipole moment of zero.
What is AsF5 hybridization?
The hybridization of As in AsF5 is sp3d. This is because there are five regions of electron density (five As-F bonds) around the As atom.
Wrapping Up
Arsenic Pentafluoride (AsF5) is interesting because it shows how a molecule’s shape affects its properties. Even though it has five polar As-F bonds, AsF5 is nonpolar because it’s shaped like a symmetrical two-sided pyramid. This shape makes the pulls of the bonds balance out, so the whole molecule has no overall pull in any direction.
We hope this article has helped you understand AsF5 better and made you curious to learn more about how molecules are shaped.


